Improving The Lives of Patients
Intro VideoAGC Biologics is a global CDMO providing development and manufacturing services for protein-based biologics and advanced therapies.
AGC Biologics is a global CDMO providing development and manufacturing services for protein-based biologics and advanced therapies.
Our Mission
To work side by side with our customers in order to improve patients’ lives by bringing new biopharmaceuticals to market.
About usOur Purpose
Our purpose is to bring hope to life by enabling life-changing therapies for patients around the globe, creating a healthier and happier tomorrow.
Your End-to-End ADC Solution
Three world-class industry leaders, including AGC Biologics, work together to streamline antibody drug conjugate projects.
On-demand webinar!
Navigating the Future
of Protein A Purification:
New tech to accelerate mAb development

Accelerate Your Project from Bench to Clinic
Antibody and LVV GMP material with rapid timelines supporting clinical goals.
Learn about our new programAGC Biologics Announces Plans for New Manufacturing Site in Yokohama, Japan
This facility will provide additional pharmaceutical development and manufacturing services to help meet the global demand for biologics and advanced therapies
Read full Press ReleaseNew research: plug-and-play vector platforms that cut CGT timelines in half
Download our article discussing how templated viral vector platforms like ProntoLVV™ and BravoAAV™ are speeding up timelines.


Our Pre-Qualified End-to-End Lentiviral Platform
Use a proven process and get to GMP in half the time!
Access our Lentiviral Platform
Templated Adeno-Associated Vector Platform
Everything you need for pre-clinical through commercial adhesion or suspension viral vector production.
Access our AAV Platform+0
Years of experience
+0
Customers served worldwide
0
Global Network Sites
+0
Commerical products
CDMO Manufacturing Capabilities
Mammalian
We have a proven track record of developing mammalian cell lines and processes from pre-clinical through commercial production.
Mammalian
Microbial
We have successfully expressed antibody fragments, growth factors, antigens, enzymes, and other proteins using multiple modes of expression.
Microbial
Cell Therapy
Our global cell therapy services are backed by 30 years of scientific expertise, and the latest systems and technology.
Cell Therapy
Viral Vectors
We are building innovative and proprietary AAV, LVV, and RVV systems that meet the latest industry needs that can support any product platform.
Viral Vectors
Plasmid DNA
Our non-GMP or GMP supply of material supports vaccine creation, RNA drugs, gene therapy and viral vector starting materials, and more.
Plasmid DNA
Messenger RNA
We offer supplies in different qualities, and development scales and have extensive in-house analytical methods for today's latest applications.
Messenger RNA
Thought Leadership Articles
Press Releases
Events & Conferences
End-to-End Development Services
Tech Transfer
Technology transfer support to partner with you at any stage in your product's lifecycle.
Tech Transfer
Process Development
We use the latest processes and technology to scale your upstream process and effectively purify your product in the downstream.
Process Development
Cell Line Development
Benefit from our advanced technologies and extensive experience in building stable and reproducible cell lines that can be scaled to commercial levels.
Cell Line Development
Analytical Development
Our comprehensive analytical testing supports any stage to ensure your product's identity, safety, purity, and potency.
Analytical Development
Formulation Development
We develop formulations for drug substance, drug product and lyophilized drug product, and provide broad, rapid excipient screening studies.
Formulation Development
Process Validation
Our well-defined approach with supporting quality systems provide you a targeted path through late-phase process validation.
Process Validation
cGMP Manufacturing
Our scales and services grow with your project, pre-clinical through full-scale commercialization.
cGMP Manufacturing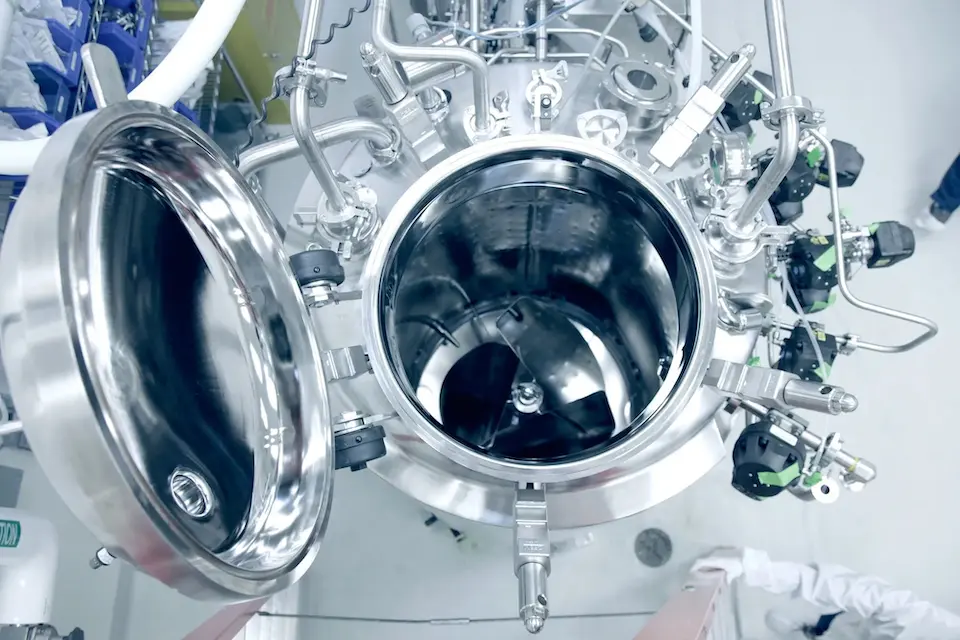
Quality Systems & Inspection Management
Our strict quality systems support cGMP manufacturing efforts to help ensure compliance with major regulatory agencies worldwide.

Environment,
Health & Safety
Responsible stewards of Our Planet
We are committed to meeting the needs of the environment by operating ethically and responsibly.
Our Global Network
Seven Sites.
Three Continents.
One Quality System.
End-to-End Development Services
Tech Transfer
Single line of text here about our service
Process Development
Single line of text here about our service
Cell Line Development
Single line of text here about our service
Analytical Development
Single line of text here about our service
Formulation Development
Single line of text here about our service
Process Validation
Single line of text here about our service
cGMP Manufacturing
Single line of text here about our service
Quality Systems and Inspection Management
Single line of text here about our service
Interested in joining our team?
We're hiring across the world. Check out our careers page to learn more and see current openings.
Watch
Explore Our Production & Manufacturing Services
"It starts with a molecule and an idea, but in the right hands, it can become so much more."

















